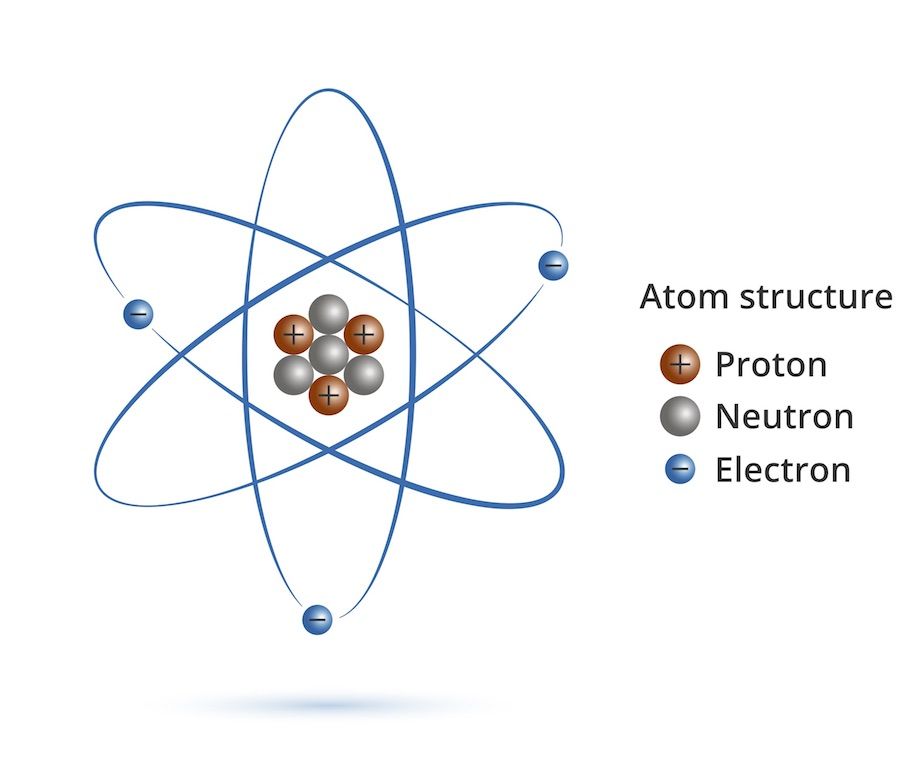
The
atom is the basic unit of matter which is the smallest thing that can
have a chemical property. Atoms are made up of two types of hadron
particles; the protons (positively charged particles), the neutrons
(particles with no charge) and one type of lepton particles called the electrons (negatively charged
particles).
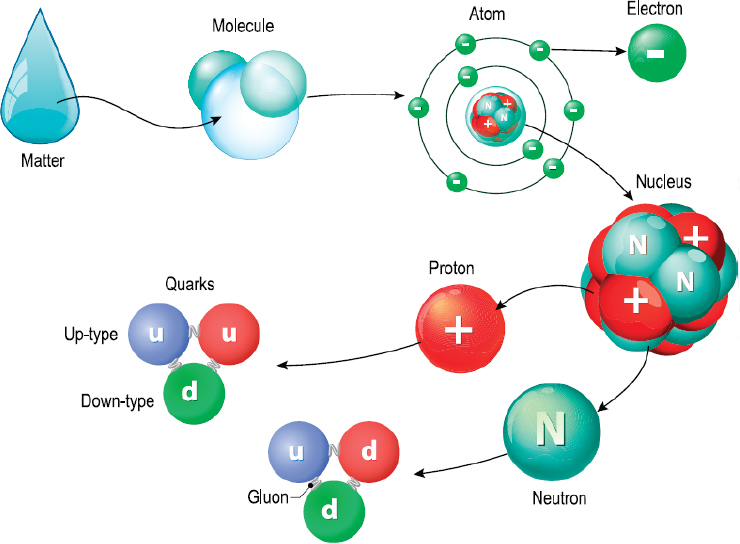
The
protons and the neutrons are heavier and remain stay at the middle of
the atom as a nucleus of the atom. But usually in nature, like
charges or same charged particles repel each other, then how the
positively charged protons in the nucleus remain stayed together?
This was solved by finding a particle called Gluon. Like as glue,
gluons act like atomic glue by sticking the protons together using
the strong nuclear force. It is this force which also holds the
quarks together that make up the protons and neutrons. The nucleus is
surrounded by a cloud of electrons which are very lightweight. The
electrons have the negative charge and the nucleus have the positive
charge, so they attract each other and the electrons orbit or travel
around the nucleus by the electromagnetic force.
There
are some atoms which have unstable nucleus such that the nucleus is
either too big to hold itself together or has too many protons or
neutrons to hold. In such cases, the nucleus has to get rid of such
excess mass or particles which has been through radiation and the
atoms whose nuclei undergo such radiation are called radioactive
atoms. The unstable atoms continue to be radioactive until they lose
enough mass/particles that they become stable. There are three types
of radioactive decay:
Alpha
Decay

Alpha
decay happens when an atom is too big and need to get rid of some
mass by shooting out a particle having two protons and two neutrons;
that is a helium nucleus. This shooting out a helium nucleus
resulting an element with atomic number two less than before.
Beta
Decay

Beta
decay happens when an atom has either too many protons or too many
neutrons and need get rid of either of two ways; when a neutron turns
into a proton (Beta-minus decay) or when a proton turns into a neutron (Beta-plus decay). When a neutron
turns into a proton, the atom shoots out an electron that resulting
to an element with one higher atomic number than before. Or when a
proton turns into a neutron, the atom shoots out a positron that
resulting to an element with one lower atomic number than before.
Gamma
Decay
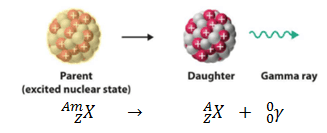
Gamma
decay is when an atom shoots out a gamma ray or wave. It happens when
there is a change in the energy of the nucleus which had already gone
through alpha or beta decay. After a nucleus undergone through alpha
decay or beta decay, it is usual that there is no change in the mass
or atomic number or the atom; but there is a change only in the
stored energy inside the nucleus.
Comments
Post a Comment